What is the medical device software test?
Verify whether the total life cycle designing, development, and verification based on Risk management document are in conformance with the test and evaluation management criteria.
What is the medical device software?
The software that is developed to serve as medical devices, including stand-alone software, embedded software, and mobile medical applications, etc.
- Stand-alone software: The computing that provides virtual information technology as a service using Internet technology.
- Embedded software: The medical device software that is operated as embedded in a separate medical device.
- Mobile medical app: A mobile app that is in accordance with the definition of medical devices (Article 2 of the Medical Device Act)
Related basis (standard)
- The requirements as per Section 14 (Programmable medical electricity system) of the "Shared Standards and Evaluation Methods for Electrical and Mechanical Safety of Medical Devices (Appendix 1 of the Announcement by the MFDS)
- IEC60601-1 (Ed.3.0/3.1) Section 14: The requirements for medical device software
- IEC62304 (Ed.1.0/1.1): The life cycle requirements of software development of medical devices
※ IEC 60601-1:2012 (3.1), Section 14, PEMS makes Sections 4.3, 5, 7, 8, and 9 of IEC 62304:2006 mandatory.
※ The importance of the medical device software validation (IEC 62304): Required for a permit in Europe (CE)
Background
The medical device software test report issued by the Information Security Center can be used in the test for the conformity of the software of the medical devices manufactured or imported at KTC, according to the Medical Device Act.
※ KTC is a certified Evaluation agency for medical device Evaluation, evaluation of technical documents, and quality conformity Evaluation.
How to evaluate the Evaluation tasks
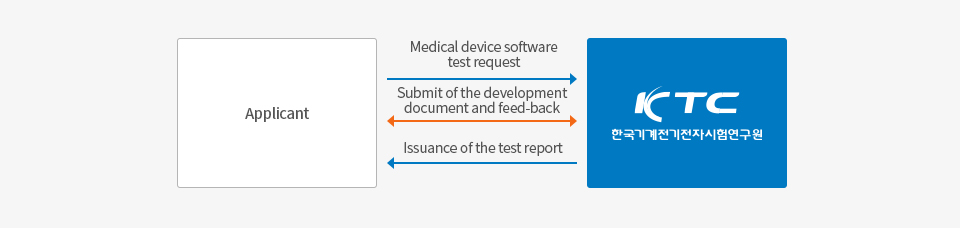
A guide to the Evaluation service evaluation
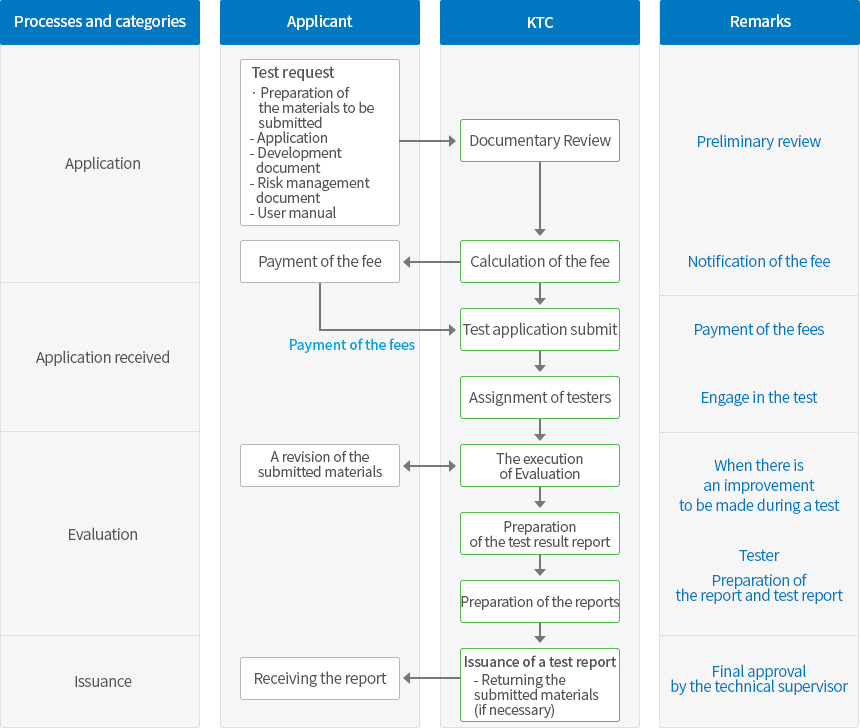
The application scope of medical device software Evaluation
| The scope of the test |
- The software used for medical purposes, software medical devices that run hardware, and the medical devices that are solely composed of software
- All software that affects the production and quality of medical devices
|
| Targets |
- The embedded software
- Software as a part of the system
- Stand-alone software
- Self-developed software
- Purchased software
- The software that is used in production or the quality system
- Digital records
|
Inquiry and Contact Information
Visit the website of the responsible department
Click the button below to jump to the main webpage of the responsible department, where you can find the contact information of the officers in charge.